Office of Communications & Media Relations
Clinical Center 1953 to Present
1940 – 1949
1950 – 1959
1960 – 1969
1970 – 1979
1980 – 1989
1990 – 1999
2000 – 2009
2010 – 2019
2020 – Present
1940 - 1949
- 1944
-
July 1, 1944
Public Law 78-410, the Public Health Service Act, authorizes establishment of the Clinical Center.
- 1947
-
July 8, 1947
Under Public Law 80-165, research construction provisions of the Appropriations Act for Fiscal Year 1948 provides funds "For the acquisition of a site, and the preparation of plans, specifications and drawings, for additional research buildings and a 600-bed clinical research hospital and necessary accessory buildings related thereto to be used in general medical research... ."
- 1948
-
1948
Dr. Jack Masur serves as the Clinical Center director. He specialized in hospital administration, planning and construction. As the NIH Clinical Center director, he helps plan the 540-bed Clinical Center.
 A 1948 aerial view of the NIH Clinical Center's footprint as construction begins shows a Lorraine cross.
A 1948 aerial view of the NIH Clinical Center's footprint as construction begins shows a Lorraine cross.November 1948
Construction on the Clinical Center starts.
1950 - 1959
- 1951
-

President Harry S. Truman (far right) lays the cornerstone for the NIH Clinical Center June 22, 1951.
1951
Dr. John A. Trautman serves as the Clinical Center director.
June 22, 1951
The cornerstone ceremony is officiated by Oscar R. Ewing, Federal Security Administrator. President Harry S. Truman is the honored guest and lays the cornerstone.
- 1953
-
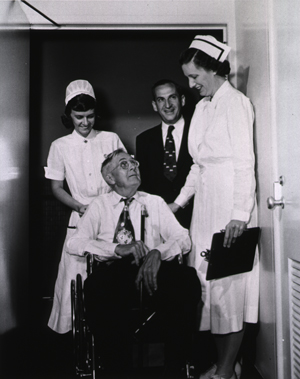
Charles Meredith is the first patient admitted to the NIH Clinical Center July 6, 1953. July 2, 1953
Department of Health, Education and Welfare Secretary Oveta Culp Hobby dedicates the Clinical Center.
July 6, 1953
Dr. Roy Hertz admits Charles Meredith, a Maryland Farmer with prostate cancer, as the first patient of the Clinical Center.
- 1954
-
1954
The Clinical Center's diagnostic x-ray department acquires the only Schnonander angiocardiographic unit in the US. It takes films in two planes at the rate of six films per second, permitting a graphic demonstration of contrast substances as they pass through the heart, making diagnosis faster and more accurate.
Dr. Donald W. Patrick serves as the Clinical Center director.
- 1956
-

Dr. Jack Masur was the NIH Clinical Center's first director from 1948 – 1951 and again from 1956 to his death in 1969. 1956
Dr. Jack Masur serves as the Clinical Center director for a second time.
- 1957
-
1957
The Clinical Pathology Department starts an approved residency training program, admitting its first two residents. The department develops the first automated machine for counting red and white blood cells (until then counted manually), from which later comes the Coulter counter.
1957
The Clinical Center Blood Bank publishes its first research paper, delineating the post-transfusion hepatitis problem, firing the first salvo in a long but largely successful campaign.
- 1959
-
1959
The circular surgical wing 10A begins construction, adding 45,000 square feet.
1960 - 1969
- 1963
-

Building 10A, which housed the cardiac and neurosurgery research facilities and associated laboratories and the NIH Blood Bank, opens in 1963. 1963
The Clinical Center Blood Bank moves to 10A, also called the "fish bowl." Blood collections begin on the NIH campus.
September 5, 1963
Dr. Luther L. Terry, surgeon general, dedicates a new surgical wing for cardiac and neurosurgery.
- 1964
-
1964
Drs. Harvey Alter, Clinical Center, and Baruch Blumberg, NIDDK, codiscover the Australian antigen, which Blumberg later shows to be the surface coating of the hepatitis B virus, leading to the isolation of this medically important virus. Alter does pioneering work in the causes and prevention of blood-transmitted infections, which helps lead to the discovery of the virus that causes hepatitis C and the development of screening methods that will reduce the risk of transfusion-transmitted hepatitis.

Dr. John L. Doppman, 1928-2000, a diagnostic and interventional radiologist at the NIH Clinical Center for 36 years, was also chief of the Clinical Center's Diagnostic Radiology Department for 26 years. 1964
Diagnostic radiologist Dr. John L. Doppman and associates in diagnostic radiology report the first successful imaging of the arteries that supply the spinal cord. The technique of spinal angiography makes surgical intervention possible where spinal arterial malformations, lesions, or tumors cause paralysis.
- 1965
-
1965
The Clinical Pathology Department acquires a Control Data 3200 computer, which fills a room the size of a small living room. Some instruments are placed online; other data are entered on key-punched cards. CPD begins using computers to manipulate lab data and report test results.
- 1966
-
1966
The Department of Nuclear Medicine, headed by Dr. Jack Davidson, is established at the Clinical Center to centralize imaging facilities for patients in any institute. Radiation Safety, Diagnostics and the Whole Body Counter Division become part of Nuclear Medicine and the old Radiation Safety Division is abolished. President Lyndon B. Johnson visits the new department.
 This is the south side of the NIH Clinical Center in the 1960s.
This is the south side of the NIH Clinical Center in the 1960s. 1966
Additions to the Clinical Center, a library and a cafeteria, begin.
- 1968
-
1968
Diagnostic radiologist Dr. John L. Doppman develops a method for locating the parathyroid, a group of glands each about the size of a BB pellet that regulates calcium metabolism.
1968
The first cancer patient enters the laminar flow room, a unidirectional air flow type of cleanroom, on 13 East.
- 1969
-
July 2, 1969
A dedication ceremony is held to name the Clinical Center's Jack Masur Auditorium.
 The Masur Auditorium is dedicated for Dr. Jack Masur July 2, 1969, after his death.
The Masur Auditorium is dedicated for Dr. Jack Masur July 2, 1969, after his death.
1970 - 1979
- 1970
-
1970
The Clinical Center Blood Bank switches to an all-volunteer donor system, adding a test for hepatitis B surface antigen. Those two measures alone reduce the hepatitis rate from 30 percent before 1970 to about 11 percent after. Later, when it adds more sensitive tests for hepatitis B, hepatitis B virtually disappears as a problem in the Blood Bank.
1970
Dr. Thomas C. Chalmers serves as the Clinical Center director.
- 1972
-
1972
Clinical Pathology's Dr. Richard B. Friedman develops a computer program to teach students to diagnose illnesses by having the computer report symptoms, inform on test availability and cost, test results and reactions to treatment.
1972
Clinical Center Blood Bank scientists develop a test for AU antigen–agent associated with hepatitis. The test will be used nationally.
- 1974
-
1974
Dr. Robert S. Gordon, Jr. serves as the Clinical Center director.
- 1976
-
1976
Dr. Mortimer B. Lipsett serves as the Clinical Center director.
- 1977
-
1977
The Clinical Center Blood Bank establishes therapeutic apheresis/exchange programs that for decades will improve the lifespan and welfare of patients with such illnesses as sickle cell disease, hyperlipidemia, and autoimmune disorders. It also establishes the first automated platelet-pheresis center, collecting platelets for transfusion from volunteer donors using automated instrumentation.
April 1977
Construction of the ambulatory care research facility begins.
 By 1978, the foundation has been laid and a portion of the wing that will be Building 10C, the Ambulatory care center, has been built.
By 1978, the foundation has been laid and a portion of the wing that will be Building 10C, the Ambulatory care center, has been built.
- 1979
-
November 1979
The Critical Care Medicine Department is established.
1980 - 1989
- 1980
-
December 12, 1980
Senate Joint Resolution 213 designates the Clinical Center as the "Warren Grant Magnuson Clinical Center of the National Institutes of Health."
- 1981
-
1981
As part of the design for the new ACRF, Clinical Pathology services - previously scattered - are brought under one roof working together in one vast open room, except for specialized functions such as the containment of radionuclides that are sequestered for safety purposes.
June 1981
The first AIDS patient is admitted.
October 1981
The ambulatory care research facility is dedicated. The research hospital is renamed the Warren Grant Magnuson Clinical Center.
- 1982
-
1982
A new surgical facility opens on the second floor of the ACRF, with more space for equipment, larger operating suites, two viewing galleries, and better delivery systems. Surgical Services performs more than 2,000 cancer, eye and general surgical procedures a year. A surgical intensive care unit opens in conjunction with new surgical suites. Nurses in the new nursing unit face new challenges in caring for patients in septic shock and providing such therapies as continuous veno-venous hemofiltration, hemodynamic monitoring and ventilator support.
September 20, 1982
The National Institute of Aging's Laboratory of Neurosciences is dedicated.
- 1983
-

Dr. John L. Decker is the director of the NIH Clinical Center from 1983 to 1990. 1983
Clinical Pathology creates an immunology service, reflecting growing demand for sophisticated antibody and cellular-level diagnostic services.
1983
Dr. John L. Decker serves as the Clinical Center director.
- 1984
-
1984
Clinical Center Blood Bank is renamed the Department of Transfusion Medicine because its activities extend well beyond traditional blood banking. DTM achieves the first transmission of HIV - HTLV III - to a primate through transfusion and describes the HIV seronegative window.
March 22, 1984
The first magnetic resonance imaging unit becomes operational for patient imaging.
October 1984
The National Cancer Institute's Radiation Oncology building is dedicated.
- 1985
-
April 13, 1985
The first two cyclotrons were delivered to the underground facility operated by the Nuclear Medicine Department.
- 1986
-
December 2, 1986
As a charter member of the National Marrow Donor Program (NMDP), the Clinical Center signs an agreement to become one of the first donor centers participating in the NMDP.
- 1987
-

Dr. Mortimer B. Lipsett is director for the NIH Clinical Center from 1976 to 1983. September 1, 1987
NIH becomes a smoke-free agency, banning smoking in all buildings including the NIH Clinical Center.
November 20, 1987
The Lipsett Amphitheater is dedicated.
1990 - 1999
- 1990
-
1990

Dr. Saul Rosen serves as the acting Clinical Center director beginning in 1990. Dr. Saul Rosen serves as the acting Clinical Center director.
September 14, 1990
A 4-year-old patient with adenosine deaminate deficiency is the first to receive gene therapy treatment.
- 1991
1991
A thrombosis unit is established in Clinical Pathology's hematology service to help manage patients with coagulopathies. A virology section is redeveloped within Clinical Pathology's microbiology service. The original viral diagnostic unit had long since lapsed, for lack of clinical utility, but with the development of new diagnostic methodologies and new therapies, the need for such a service has become increasingly apparent.
April 8, 1991
The Department of Transfusion Medicine opens its state-of-the-art facility.
- 1992
June 1992
The A-wing addition is completed, adding National Cancer Institute and National Institute of Allergy and Infectious Diseases labs focusing on AIDS research.
- 1993
July 1993
The hematology/bone marrow unit opens to improve transplant procedures and develop gene therapy techniques.
- 1994

Dr. John I. Gallin is the director of the NIH Clinical Center from 1994 to 2017. He was the Clinical Center's longest serving director. May 1994
The first multi-Institute unit designed and staffed for children opens.
May 1, 1994
Dr. John I. Gallin serves as the Clinical Center director.
- 1995
1995
Diagnostic Radiology installs a 20,000-pound magnetic resonance scanner in the courtyard outside Transfusion Medicine.
- 1996
February 1996
Details on clinical research studies conducted at the Clinical Center are made available on the World Wide Web at http://clinicalstudies.info.nih.gov, increasing opportunities for physicians to participate in NIH clinical investigations.
September 12, 1996
House Resolution 3755, Section 218, named the new clinical research center at the National Institutes of Health as the Mark O. Hatfield Clinical Research Center.
October 1996
The Secretary of Health and Human Services, marking a new governing system for the Clinical Center, appoints a Board of Governors.
- 1997
July 1997
Department of Transfusion Medicine launches a 3,000-square feet cell processing facility, created to meet increasing investigative needs for cell products used in research into new cellular therapies such as immunotherapy, gene therapy, stem cell transplantation and pancreatic islet cell transplantation.
November 4, 1997
Vice President Al Gore and Senator Mark O. Hatfield attended groundbreaking ceremonies for the Mark O. Hatfield Clinical Research Center.
 On hand with shovels for the November 4, 1997, groundbreaking of the new Mark O. Hatfield Clinical Research Center are (from left) Jane Reese-Coulbourne, Sen. Arlen Specter, Dr. John I. Gallin, Sen. Mark Hatfield, Dr. Harold Varmus, Vice President Al Gore, Donna Shalala, Rep. John Porter and Charles Tolchin. Reese-Coulbourne and Tolchin were Clinical Center patients.
On hand with shovels for the November 4, 1997, groundbreaking of the new Mark O. Hatfield Clinical Research Center are (from left) Jane Reese-Coulbourne, Sen. Arlen Specter, Dr. John I. Gallin, Sen. Mark Hatfield, Dr. Harold Varmus, Vice President Al Gore, Donna Shalala, Rep. John Porter and Charles Tolchin. Reese-Coulbourne and Tolchin were Clinical Center patients.
- 1999
1999
Clinical Pathology Department is renamed Department of Laboratory Medicine. A new laboratory information system is put in place for Laboratory Medicine, Transfusion Medicine, and the Pathology Lab.
1999
The new South Entry to the Clinical Center opens to facilitate construction on the Mark O. Hatfield Clinical Center on the north face of Building 10.
2000 - 2009
- 2000
-
2000
The National Institute of Diabetes, Digestive and Kidney Diseases and the Clinical Center, in collaboration with Walter Reed Army Medical Center, the Naval Medical Research Center and the Diabetes Research Institute of the University of Miami, launch a new kidney, pancreas and islet transplant program. The idea is to test novel therapies that may eliminate the need for the immunosuppressive drugs patients take to keep their bodies from rejecting new transplanted organs. Soon after the program starts, Dr. Allan Kirk performs the NIH's first successful kidney transplant procedure and Dr. David Harlan performs one of the first successful islet allotransplants in the United States.
2000
Clinical Center and National Institute of Allergy and Infectious Diseases scientists demonstrate that the widely used herbal product St. John's Wort could significantly compromise the effectiveness of a protease inhibitor often used to treat those infected with HIV.
2000
Clinical Center launches a new Pain and Palliative Care Consult Service.
September 22, 2000

Dr. Harvey Alter, chief of the Infectious Disease section and associate director of the research in the Department of Transfusion Medicine, receives a Laskar Award for clinical medical research medical research September 22, 2000. Dr. Harvey Alter, Department of Transfusion Medicine, receives the Lasker Award "for pioneering work leading to the discovery of the virus that causes hepatitis C and the development of screening methods that reduced risk associated with transfusion-associated hepatitis in the United States from 30 percent in 1970 to virtually zero in 2000." Alter, who is also elected to the National Academy of Sciences, shares the award with Chiron's Michael Houghton.
2000
The Imaging Sciences Program takes first steps toward filmless radiology, unveiling the pilot phase of its new Picture Archiving and Communication System (PACS) and Radiology Information System (RIS). RIS is a sophisticated patient tracking system, which will track patient arrival and departure times, the start and end of exams, and when reports are dictated, read, and signed. It is expected to reduce patient waiting times, improve image availability, and minimize loss and misidentification of images and reports. Images stored in PACS/RIS originate from procedures and exams conducted in the Diagnostic Radiology, Nuclear Medicine, and PET Departments. They include CT scans, MR scans, PET scans, nuclear medicine scans, ultrasound examinations, and digital radiography examinations.
- 2001
-
2001
A second bone marrow transplant unit opens to support NCI protocols.
- 2002
-
2002
The Department of Transfusion Medicine establishes a model program for collecting blood from subjects with hereditary hemochromatosis. This program supplies 10% of the hospital's red cell needs.
October 28, 2002
Dr. Roy Hertz, 93, a renowned NCI researcher who admitted the first patient to the NIH Clinical Center in 1956, dies. Hertz received an Albert Lasker Medical Research Award in 1972 for an early effective drug treatment for a cancerous tumor.
October 29, 2002
A groundbreaking ceremony is held for the Edmond J. Safra Family Lodge at NIH. Located steps away from the Mark O. Hatfield Clinical Research Center, the lodge will provide a comfortable home away from home for the families and caretakers of Clinical Center patients.
 Breaking ground for the Edmund J. Safra Family Lodge are (from left): Dr. Michael Gottesman, deputy director for the NIH Intramural Research Program; Dr. Elias Zerhouni, NIH director; Dr. John I. Gallin, NIH Clinical Center director; Jeffrey Keil, Ellesse LLC president; Amy McGuire, Foundation for NIH executive director; and Susan Lowell Butler, a member of the NIH Clinical Center Patient Advisory Group.
Breaking ground for the Edmund J. Safra Family Lodge are (from left): Dr. Michael Gottesman, deputy director for the NIH Intramural Research Program; Dr. Elias Zerhouni, NIH director; Dr. John I. Gallin, NIH Clinical Center director; Jeffrey Keil, Ellesse LLC president; Amy McGuire, Foundation for NIH executive director; and Susan Lowell Butler, a member of the NIH Clinical Center Patient Advisory Group.
- 2003
-
March 2003
In response to the president's request for voluntary smallpox vaccination of healthcare workers, the Clinical Center initiates a voluntary smallpox vaccination program for specified Clinical Center healthcare workers.
June 2003
The Clinical Center establishes the new Office of Clinical Research Training and Medical Education. Rapid growth and the need to expand clinical research training opportunities at the Clinical Center, and for NIH intramural scientific research programs in general, spurred establishment of the new office.
July 2003
The NIH Clinical Center marks its 50th anniversary.
- 2004
-
August 21, 2004
CRIS, the Clinical Research Information System, designed to replace the current Medical Information System, is launched.
September 22, 2004
A dedication ceremony is held for the Mark O. Hatfield Clinical Research Center, the 870,000-square-foot addition the NIH Clinical Center, the world's largest facility dedicated to clinical research.
- 2005
-

Dr. John I. Gallin welcomes patient Marcos Arrieta to the pediatrics unit in the new Hatfield Clinical Research Center April 2, 2005. Arrieta was the first pediatric patient to make the move into the new building. April 2, 2005
Current patients move to the new Mark O. Hatfield Clinical Research Center. The facility houses improved environments both for clinical research and for patient care.
April 3, 2005
A Vermont resident devoted to helping others becomes the first patient admitted to the Mark O. Hatfield Clinical Research Center.
June 1, 2005
The Edmond J. Safra Family Lodge opens. Its 34 guest rooms provide a comforting home away from home for families and caregivers of Clinical Center patients who come to NIH to participate in clinical research.
- 2006
-
June 2006
Members of the Department of Laboratory Medicine's microbiology service collaborate with National Institute of Allergy and Infectious Diseases staff to discover and name a new bacterium. The bacterium is a novel member of the Acetobacteraceae family. This prevalent family of bacteria had not been associated with human disease.
- 2007
-

At the opening of the new Metabolic Clinical Research Unit at the NIH Clinical Center January 25, 2007, are (from left): Terri Wakefield, director of clinical research services for core intramural NIDDK; Dr. Griffin Rodgers, acting NIDDK director; Dr. John I. Gallin, NIH Clinical Center director; Dr. Marvin Gershengorn, NIDDK intramural research scientific director; Dr. Monica Skarulis, senior clinical investigator with NIDDK Clinical Endocrine Branch; Dr. Elias Zerhouni, NIH director; and Lorie Purdie, Clinical Center Inpatient Services division nurse manager. January 25, 2007
State-of-the art metabolic clinical research unit opens, enabling researchers from across NIH to study factors that contribute to obesity and associated diseases.
May 2007
The Clinical Center enrolls its first patient in the human genome sequencing study–the first of 1,000 participants to enroll in a study led by NHGRI to test the use of human genome sequencing in a clinical research setting. The study uses DNA sequencing to learn whether tiny changes in selected genes indicate predisposition to or onset of common diseases.
- 2008
-
May 19, 2008
Undiagnosed Diseases Program is launched, bringing patients to the Clinical Center who seek renewed hope for puzzling, often devastating, health conditions.
June 18, 2008
The Department of Transfusion Medicine dedicates the Charles S. Carter Cellular Therapy Laboratory within their Cell Processing Section (CPS). Carter, who died in 2006, co-founded the cell processing laboratory and worked as a biologist, technologist and supervisor in DTM for 24 years.
November 10-15, 2008
NIH clinician-scientists take the Clinical Center's course, "Introduction to the Principles and Practice of Clinical Research," to Beijing, China, providing new opportunities for training in clinical research.
- 2009
-
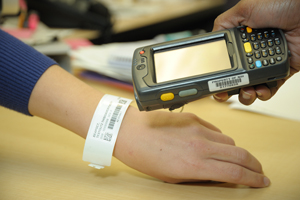
In 2009, barcodes are initially used to enhance specimen collection activity, with plans to integrate barcodes into the blood and medications administration processes in 2010.
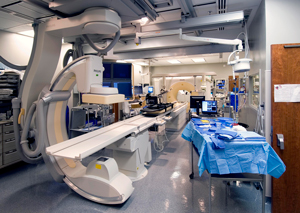
The Center for Interventional Oncology showcases an "operating room of the future" in May 2009. The OR included rotational angiography in the foreground and CT-based tools in the background. March 30, 2009
Barcode technology is initiated to enhance patient safety and improve research specimen collection.
May 2009
The Center for Interventional Oncology is created, a partnership between Radiology and Imaging Sciences and both the National Cancer Institute and the National Heart, Lung and Blood Institute for the Center for Interventional Oncology. The center will investigate how imaging technology can diagnose and treat localized cancers in ways that are precisely targeted and minimally or non-invasive.
July 30, 2009
BTRIS, the Biomedical Translational Research Information System activated. The launch of the NIH-wide research data repository allows principal investigators with active protocols to view their patients' identified data from the Clinical Center's Clinical Research Information System, the Clinical Research Information Management System of NIAID, and the NIAAA database. By September NIH researchers will be able to access de-identified patient data from the same systems.
September 2009
Radiology and Imaging Sciences teams with National Institute of Allergy and Infectious Diseases for the Center for Infectious Disease Imaging. This trans-NIH partnership will use state-of-the-art imaging methods to combat public health threats such as influenza, severe acute respiratory syndrome (SARS), poxviruses and other emerging pathogens.
2010 - 2019
- 2010
-
January 8, 2010
State-of-the-art pharmaceutical development facility opens to formulate candidate drugs.

The Special Clinical Studies Unit is an inpatient unit designed with state-of-the-art infrastructure that allows for isolation capabilities and infection control while patients participate in clinical research studies. April 14, 2010
Seven-bed Special Clinical Studies Unit opens, with advanced isolation and extended-stay capabilities.
June 2010
Edmond J. Safra Family Lodge celebrates its fifth anniversary.
September 2010
The NIH Clinical Center launches its pilot program in partnership with Project SEARCH, an international organization, for a 30-week unpaid internship to provide opportunities for young adults with disabilities to learn employability skills and gain work experience.
- 2011
-
January 2011
A new pilot partnership between the Clinical Center, the National Cancer Institute's Center for Cancer Research and the Damon Runyon Cancer Research Foundation offers some of the capabilities and expertise of the CC to an outside group of clinical investigators in cancer research.
September 23, 2011
The Clinical Center is named the 2011 recipient of the Lasker~Bloomberg Public Service Award from the Albert and Mary Lasker Foundation, an organization which has recognized outstanding advances in medical research each year since 1945. Dr. John I. Gallin, CC director, accepted the award on behalf of the CC at the annual recognition ceremony hosted by the Albert and Mary Lasker Foundation in New York City on September 23. The award honors the CC for serving as a model institution that has transformed scientific advances into innovative therapies and provided high-quality care to patients.
 Maria Freire (left) Lasker Foundation president, watches as Dr. John I. Gallin (second from left), NIH Clinical Center director, accepts the 2011 Lasker~Bloomberg Award on behalf of the NIH and Clinical Center the award from Michael Bloomberg (center), New York City mayor and the award's namesake. Alfred Sommer (second from right), Lasker Foundation chair; and Dr. Harvey Fineberg (right), Institute of medicine president and 2011 Lasker Foundation Public service Award Committee chair were also there for the presentation.
Maria Freire (left) Lasker Foundation president, watches as Dr. John I. Gallin (second from left), NIH Clinical Center director, accepts the 2011 Lasker~Bloomberg Award on behalf of the NIH and Clinical Center the award from Michael Bloomberg (center), New York City mayor and the award's namesake. Alfred Sommer (second from right), Lasker Foundation chair; and Dr. Harvey Fineberg (right), Institute of medicine president and 2011 Lasker Foundation Public service Award Committee chair were also there for the presentation.
- 2012
-
February 2012
Clinical Center joins ResearchMatch, which connects researchers with interested and eligible volunteers.

NIH Clinical Center medical technologist Minh Tran thaws and processes a bone marrow stromal cell unit for infusion to a patient March 15, 2012. March 15, 2012
The Clinical Center treated its first research study volunteer with an infusion of bone marrow stromal cells (BMSC), which are cells that help to regulate the immune system.
August 2012
With NIH, the CC opens pathways for collaborations between intramural and extramural investigators. The recommendation that the NIH allow external investigators to use the unique resources of the CC to stimulate a broader range of research came from the Congressionally mandated Scientific Management Review Board (SMRB).
- 2013
-

At the Samuel J. Heyman service to America Medals Ceremony in October 2013 are (from left): Denis McDonough, White house chief of staff; Drs. Julie Segre, Evan Snitkin, Tara Palmore and David Henderson, all with the NIH Clinical Center. October 2013
Dr. Julie Segre, Dr. Evan Snitkin, Dr. Tara Palmore, and Dr. David Henderson earn the title "Federal Employees of the Year" for their breakthrough in tracking and controlling of a cluster of antibiotic-resistant bacterial infections at the Clinical Center with the use of DNA sequencing.
October 24, 2013
Dr. Harvey J. Alter, M.D., infectious disease researcher and clinician, and chief of clinical studies and associate director of research in the Department of Transfusion Medicine, receives the 2013 Canada Gairdner International Award. Dr. Alter shares the award with Daniel Bradley, Ph.D., consultant at the Centers for Disease Control and Prevention, and Michael Houghton, Ph.D., researcher and professor at the University of Alberta, Edmonton, Canada, for their critical contributions to the discovery and isolation of the hepatitis C virus, which has led to development of new diagnostic and therapeutic agents.
July 2013
The Clinical Center celebrates its 60th anniversary.
- 2014
-
January 2014
Bedside medication barcoding, a new system to help avoid medication administration errors, began as a pilot on select units. The program was fully integrated by March 2014.
March 7, 2014
His Holiness, the 14th Dalai Lama, Tenzin Gyatso, visited the NIH Clinical Center and then presents at the NIH J. Edward Rall Cultural Lecture on the importance of science in human flourishing.
 His Holiness, the 14th Dalai Lama, Tenzin Gyatso, visits the NIH Clinical Center March 7, 2014.
His Holiness, the 14th Dalai Lama, Tenzin Gyatso, visits the NIH Clinical Center March 7, 2014.March 2014
NIH announces that it will open the NIH Clinical Center to non-government researchers through three-year renewable research grants of up to $500,000 per year. The new program will allow scientists to collaborate with NIH investigators in a highly specialized hospital setting as they work toward translating promising laboratory discoveries into improved disease diagnosis, prevention, and treatment.
October 16, 2014
The NIH Clinical Center admits its first patient with the Ebola virus, which causes an acute, serious illness which is often fatal if untreated. The patient was treated in the Special Clinical Studies Unit which is specifically designed to provide high-level isolation capabilities and is staffed by infectious diseases and critical care specialists. The patient was discharged Oct. 24, declared free of the Ebola virus, after five negative PCR (polymerase chain reaction) tests.
December 2, 2014
President Barack Obama visits the Clinical Center and Vaccine Research Center to express his gratitude to Clinical Center staff for their treatment of an Ebola patient and ongoing Ebola research.
- 2015
-
June 2015
To increase the Department of Transfusion Medicine's research capabilities of cell products, the NIH Blood Bank moved all platelet and granulocyte donations from the NIH Clinical Center to its new location at 5625 Fishers Lane, Rockville, Md., 20852.
March 10, 2015
A patient undergoing stem cell transplantation is the first of 10 patients to receive a novel cell therapy treatment designed to fend off four viruses that commonly affect stem cell transplant recipients: Cytomegalovirus, Epstein-Barr virus, Adenovirus and BK-virus, as part of a National Heart, Lung, and Blood Institute clinical trial.
September 2015
The NIH Clinical Center was announced as the first federal medical facility to be recognized by Health Information and Management Systems Society Analytics. The Clinical Center was awarded the research hospital Stage 7 certification, the highest level attainable, for its electronic medical record adoption model, eliminating the use of paper charts and maintaining a superior electronic medical record system for inpatient care.
- 2016
-
January 2016
January 2016 (published date) - Researchers at the National Institutes of Health Clinical Center, in collaboration with extramural organizations, have sequenced nearly the entire genome of human, mouse and rat Pneumocystis.
March 2016
A photon-counting computed tomography (CT) scanner is used in patients for the first time at the NIH Clinical Center. The prototype technology is expected to replicate the image quality of conventional CT scanning, but may also provide health care specialists with an enhanced look inside the body through multi-energy imaging.
- 2017
-

Dr. Francis Collins (left), director of the National Institutes of Health, swears in the NIH Clinical Center's first chief executive officer, Dr. James K. Gilman, January 9, 2017. January 9, 2017
Dr. James K. Gilman serves as the first chief executive officer of the NIH Clinical Center.
January 10, 2017
The NIH Clinical Center upgrades its FollowMyHealth patient portal to improve patient engagement and satisfaction.
April 2017
The Clinical Center implements the new Safety Tracking and Reporting System, or STARS, that replaces the Occurrence Reporting System (ORS). The patient safety event reporting system will be used to track safety and quality concerns - including errors, near misses (an event that does not reach a patient but could have caused harm if it had), service quality concerns such as delays and unsafe environmental conditions, and instances of exceptional customer service by hospital staff.
August 10, 2017

Jim Parsons (left), narrator for the First in Human documentary, meets Dr. Alexandra Freeman, NIAID, while he is at the Clinical Center filming for the documentary. The first episode of "First in Human" a three-part documentary series about the NIH Clinical Center’s staff and patients airs. The program, produced by The Discovery Channel, highlights the innovation and hard work that takes place in the hospital, depicts how challenging illness are diagnosed and treated and provides an inside look at the successes and setbacks that are a part of experimental medicine.
August 2017
Researchers from the NIH Clinical Center Rehabilitation Medicine Department created the first robotic exoskeleton specifically designed to treat crouch (or flexed-knee) gait in children. Crouch gait, the excessive bending of the knees while walking, is a common and debilitating condition in children with cerebral palsy. The exoskeleton provides powered knee extension assistance to support patients at key points during the walking cycle.
August 2017
The Clinical Center releases over 100,000 anonymized chest x-ray images and their corresponding data, allowing researchers in the U.S. and around the world to freely access the datasets to detect and diagnose disease through computerized review. Ultimately, this artificial intelligence mechanism may lead to clinicians making better diagnostic decisions for patients. This is one of the largest chest x-ray datasets publicly available to the scientific community.
November 27, 2017
The Clinical Center Pediatric Observation Unit opens to provide enhanced support for patient safety in pediatric research. The unit can accommodate younger and sicker children, enhancing the hospital’s ability to care for more fragile pediatric patients. The unit has customized beds that monitor the heart, lungs, nervous system and metabolism of pediatric patients who need closer clinical observation.
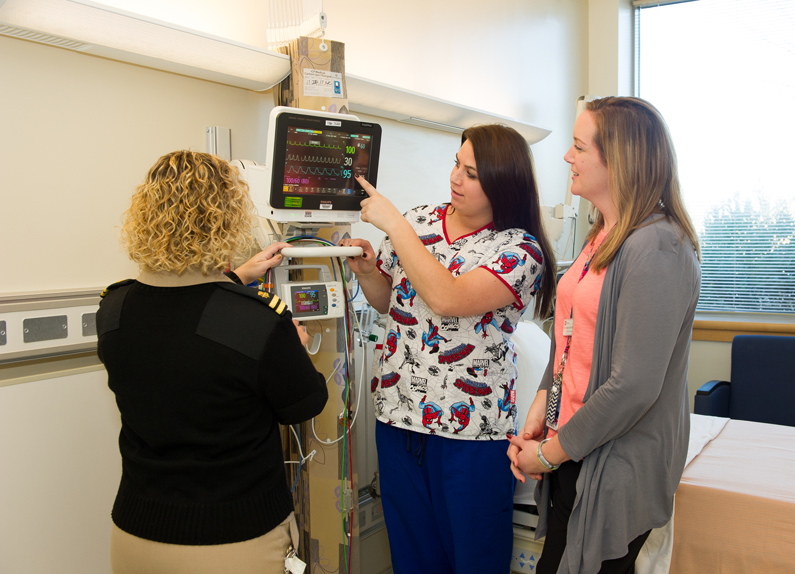 Nurses Lee Ann Keener, Kelly White and Alex Classen view the features of a new pediatric bedside monitor in one of the rooms of the new Pediatric Observation Unit Nov. 27, 2017.
Nurses Lee Ann Keener, Kelly White and Alex Classen view the features of a new pediatric bedside monitor in one of the rooms of the new Pediatric Observation Unit Nov. 27, 2017. - 2018
-
2018
The Health Information and Management Systems Society (HIMSS) Analytics re-certified the Clinical Center’s electronic health record, known as Clinical Records and Information System (CRIS), with its highest certification on the Electronic Medical Record Adoption Model, Stage 7. CRIS originally secured Stage 7 certification in 2015 and the system is used to maintain all patient care, research and administrative information.
Spring 2018
The Clinical Center patient library catalog becomes digitally accessible. NIH patients, their family members and caregivers can now go online from any computer, tablet or mobile device to browse and reserve more than 7,000 items. Previously the catalog was only accessible to people who visited the library in person.
March 1, 2018
National Institutes of Health and Inova Health System researchers launch The Genomic Ascertainment Cohort (TGAC) , a two-year pilot project to examine genes and gene variants’ influence on individual, observable traits, such as height, eye color or blood type. The TGAC’s new database of 10,000 human genomes and exomes, 1-2 percent of the genome that contains protein-coding genes, provides a directory that researchers can use to predict conditions that specific genes or gene variants might produce and test those predictions by re-examining individuals who donated their DNA sequence information. Participants will come to the NIH Clinical Center, using the hospital’s unparalleled phenotyping resources and capabilities.
April 11, 2018
Researchers at the National Institutes of Health work with 15 patients from around the world to uncover a genetic basis of “dripping candle wax” bone disease. The rare disorder, known as melorheostosis, causes excess bone formation that resembles dripping candle wax on x-rays. The condition causes pain and bone deformity, which can limit the function of bones. There are only about 400 known cases of this disorder worldwide and 15 unrelated adults with the condition volunteered to come to the NIH Clinical Center to undergo biopsies of both affected and unaffected bones. The results offer potential treatment targets for this rare disease, provide important clues about bone development and may lead to insights about fracture healing and osteoporosis.
July 10, 2018

Dr. Ann Berger, chief of the Pain and Palliative Care Service, Dr. Francis Collins, director of the NIH, Dr. Jim Gilman, CEO of the Clinical Center and Dr. Gwen Wallen, chief nursing officer, open the Hospice Suites July 10, 2018. The NIH Clinical Center opens a Hospice Unit as a part of its Medical Oncology section. The unit is comprised of two rooms that have been converted into a home-like environment where families can stay with adult patients who are nearing end of life. Each suite has a bedroom and a communal space, including a kitchen and family sitting area.
September 24, 2018
The National Institutes of Health expands expands its Undiagnosed Diseases Network (UDN) from seven to 12 clinical sites across the US. The expansion increases the geographical distribution of the nationwide network and the number of people with access to a UDN clinical site. Since opening to applications in 2015, the network has already diagnosed over 200 cases that had long been mysteries to the medical community. The UDN was launched to build on the success of the Undiagnosed Diseases Program (UDP) at the NIH Clinical Center, which was established in 2008. A national extension to the UDP was launched in September 2015 consisting of seven clinical sites, two sequencing cores, a coordinating center, with the eventual additions of a central biorepository, a metabolomics core and a model organisms screening center.
Winter 2018
The NIH Clinical Center’s Edmond J. Safra Family Lodge is renovated, incorporating new paint, updated wallpaper and an abundance of beautiful upholstery. The Lodge is a home-like place of respite for families and loved ones of adult patients who are receiving care at the NIH Clinical Center, offering overnight accommodations for families and caregivers to be nearby when their loved ones undergo evaluation and intensive medical treatment.
 A redesigned bedroom in the NIH Clinical Center Edmond J. Safra Family Lodge.
A redesigned bedroom in the NIH Clinical Center Edmond J. Safra Family Lodge.
2020 - Present
- 2020
-
October 2020

Dr. Harvey J. Alter is a Senior Scholar in the Department of Transfusion Medicine, October 2020. NIH Clinical Center researcher Dr. Harvey J. Alter wins the 2020 Nobel Prize in Physiology or Medicine for his contributions to the discovery of the hepatitis C virus. Alter, a Senior Scholar in the hospital’s Department of Transfusion Medicine, shares the award with Michael Houghton, PhD, from the University of Alberta in Canada and Charles M. Rice, PhD, from Rockefeller University in New York City. Dr. Alter’s career at NIH has spanned more than 50 years where he focused his research on the occurrence of hepatitis in patients who had received blood transfusions.
NOTE: PDF documents require the free Adobe Reader.
This page last updated on 05/31/2022.
